
Subcutaneous nanotherapy repurposes the immunosuppressive mechanism of rapamycin to enhance allogeneic islet graft viability
Jacqueline Burke1,2, Xiaoming Zhang3,4, Molly A. Frey4,5, Sharan Kumar Reddy Bobbala1, Reese AK Richardson5, Sean D. Allen5, Yu-Gang Liu1,2, Carolina Bohorquez Fuentes1,2, Helena Freire Haddad1,2, Guillermo A. Ameer1,2,4,5, Evan A. Scott1,2,4,5.
1Biomedical Engineering , Northwestern University, Evanston, IL, United States; 2Center for Advanced Regenerative Engineering, Northwestern University, Evanston, IL, United States; 3Comprehensive Transplant Center, Organ Transplant, Surgery , Northwestern University , Chicago, IL, United States; 4Feinberg School of Medicine, Northwestern University, Chicago, IL, United States; 5Interdisciplinary Biological Sciences, Northwestern University, Evanston, IL, United States
Rapamune® (oral rapamycin/sirolimus) administration is plagued by poor bioavailability, extensive fecal elimination, and wide biodistribution. Thus, this pleotropic mTOR inhibitor has a narrow therapeutic window, numerous side effects and provides inadequate transplantation protection. Parental formulation was not possible due to rapamycin’s hydrophobicity (log P 4.3). Here, we demonstrate that subcutaneous rapamycin delivery via poly(ethylene glycol)-b-poly(propylene sulfide)(PEG-b-PPS) polymersome (PS) nanocarriers modulates cellular biodistribution of rapamycin to change its immunosuppressive mechanism for enhanced efficacy while minimizing side effects (Fig. 1).
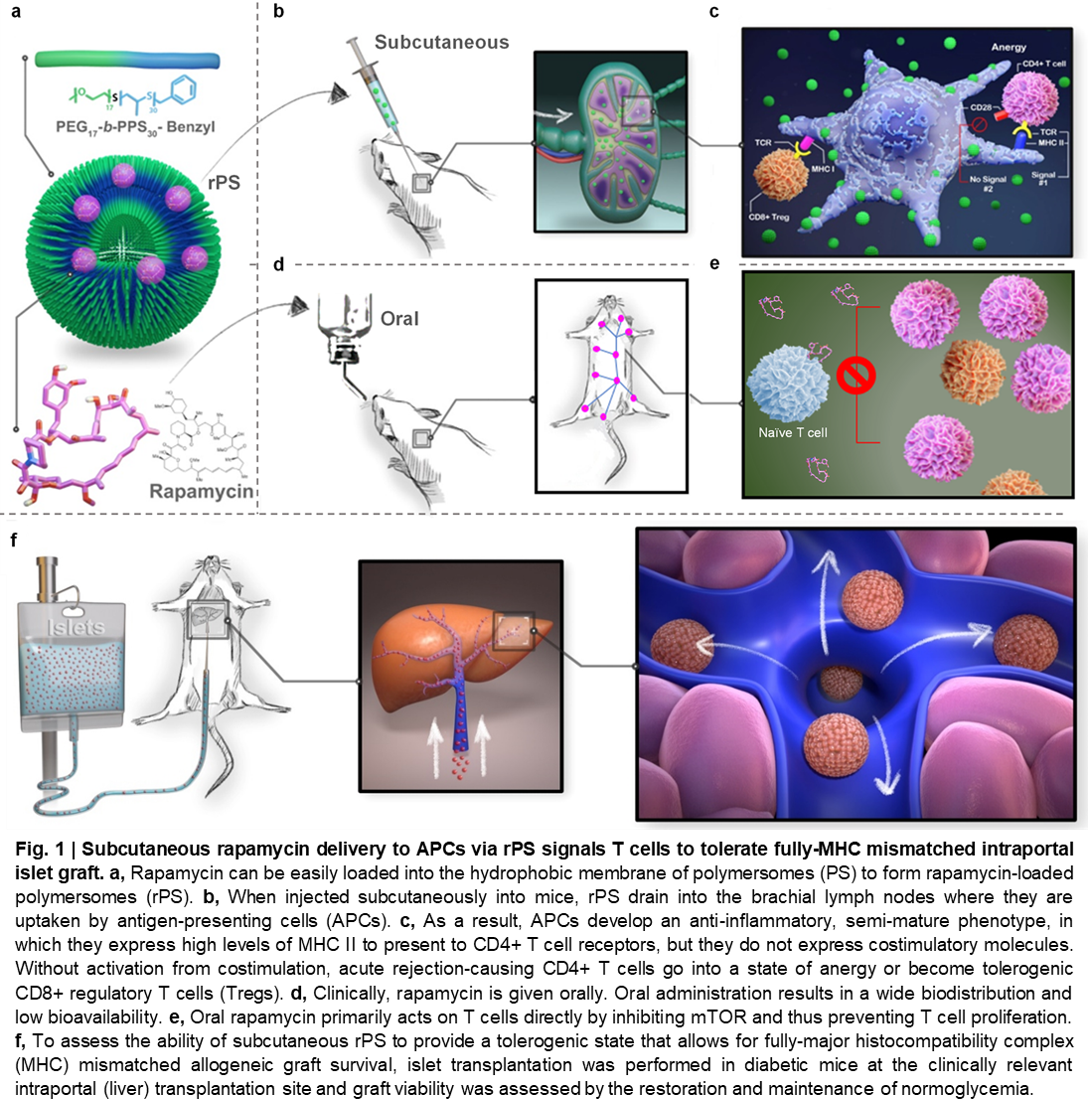
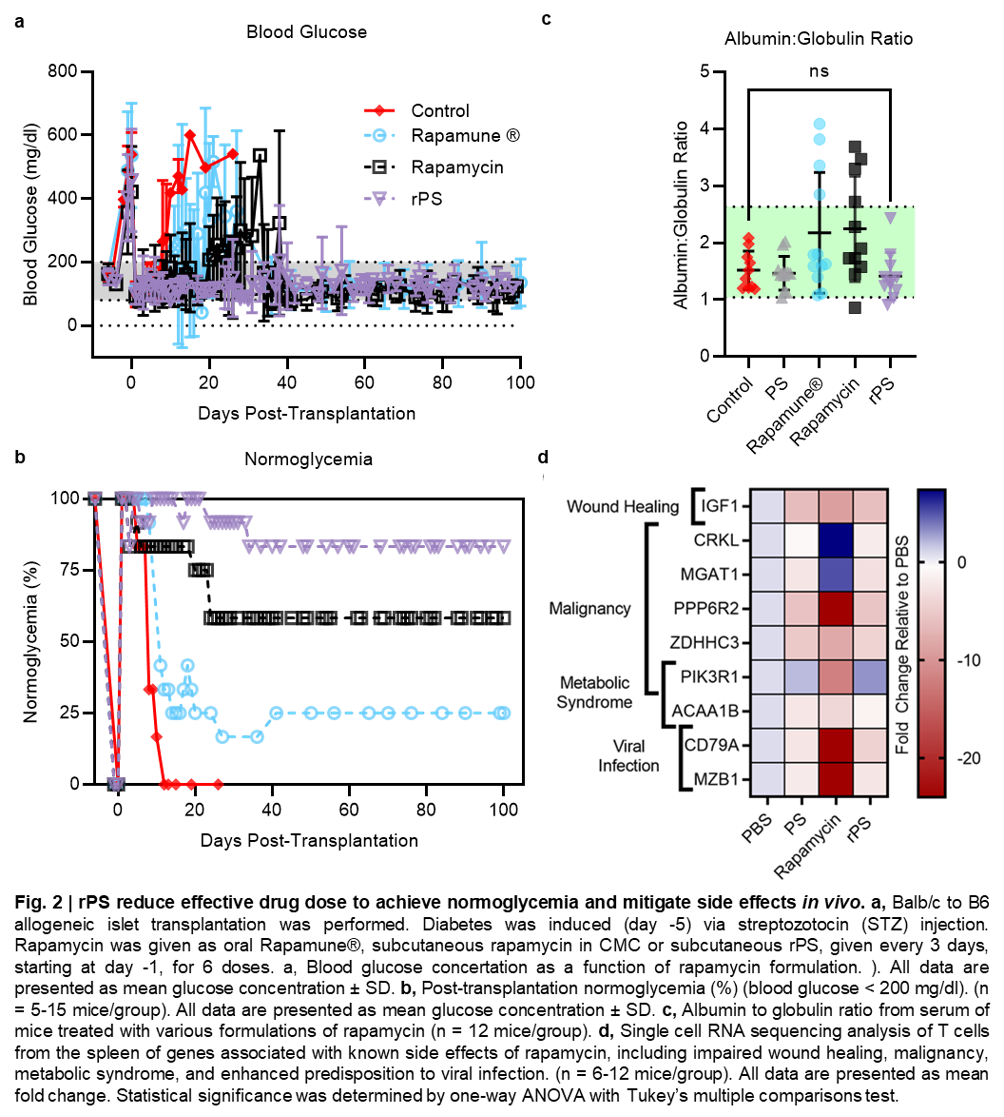
Subcutaneously administered rapamycin-loaded polymersomes (rPS) avoid fecal elimination and allow for sustained delivery of rapamycin to immune cell rich organs, especially the axillary lymph nodes. While oral rapamycin inhibits naïve T cell proliferation directly, rPS instead modulated Ly-6Clow monocytes and tolerogenic semi-mature dendritic cells, with immunosuppression mediated by CD8+ Tregs and rare CD4+ CD8+ double-positive T cells. As PEG-b-PPS PS are uniquely non-inflammatory, background immunostimulation from the vehicle was avoided, allowing immunomodulation to be primarily attributed to rapamycin’s cellular biodistribution. Repurposing mTOR inhibition significantly improved maintenance of normoglycemia in a clinically relevant, MHC-mismatched, allogeneic, intraportal (liver) islet transplantation model (Fig. 2a,b). Importantly, rPS treatment does not alter serum albumin:globulin ratios (Fig. 2c).
These results demonstrate the ability of engineered nanocarriers to repurpose drugs for alternate routes of administration by rationally controlling cellular biodistribution.