Autologous regulatory T cells in clinical intraportal allogenic pancreatic islet transplantation
Marcus Bergström1, Ming Yao2,3, Malin Müller1, Olle Korsgren1, Bengt von Zur-Mühlen4, Torbjörn Lundgren2,3.
1Department of Immunology, Genetics and Pathology, Rudbeck Laboratory, Uppsala University, Section of Clinical Immunology, Uppsala, Sweden; 2Department of Clinical Science, Intervention and Technology, Karolinska Institute, Transplantation Surgery, Stockholm, Sweden; 3Department of Transplantation Surgery, Karolinska University Hospital, Karolinska, Transplantation Surgery, Stockholm, Sweden; 4Department of Surgical Sciences, Uppsala University, Transplantation Surgery, Uppsala, Sweden
Intro
Allogeneic islet transplantation in type 1 diabetics requires immunosuppression to prevent graft rejection. These medications may cause toxicities and increases the susceptibility for infections and malignancies. Adoptive therapies with Regulatory T cells (Tregs) have shown promise in reducing the need for immunosuppression in several human transplantation settings, but have previously not been evaluated in islet transplantation. This first-in-man study of autologous intra-portal Treg infusion during allogenic pancreatic islet transplantation explores the safety and feasibility of this novel procedure.
Methods
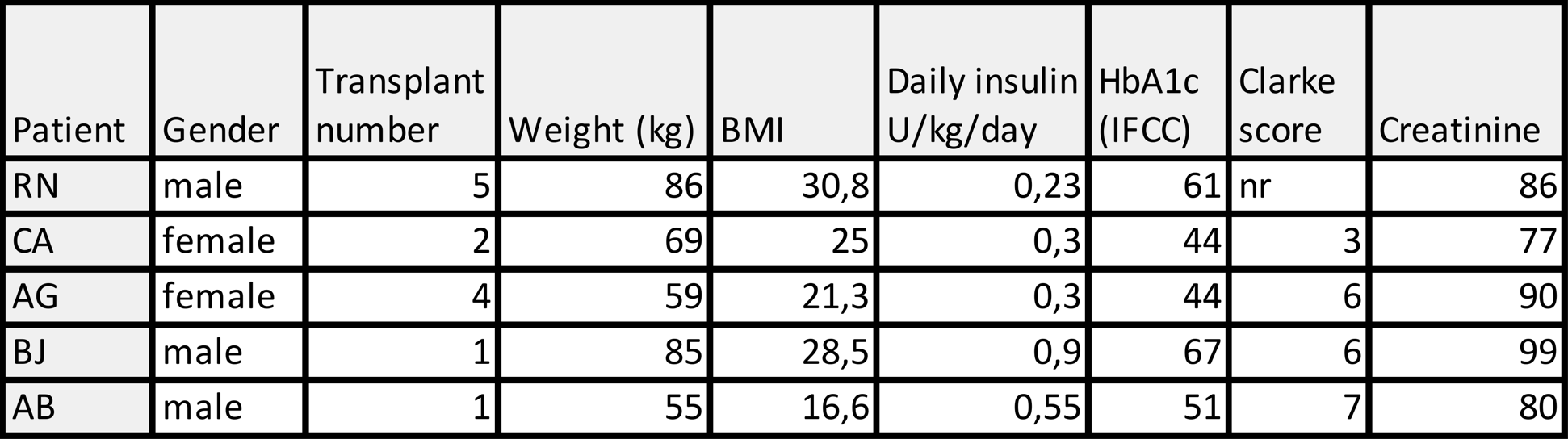
Five patients with type 1 diabetes undergoing intra-portal allogeneic islet transplantation were co-infused with polyclonal autologous Tregs under a modified Edmonton immunosuppressive regimen. While on the waiting list, patients underwent a leaukapheresis from which Tregs were purified by magnetic-activated cell sorting (MACS): first by depletion of CD8 and CD19 positive cells, then by enrichment of CD25 positive cells. Tregs were cryopreserved until transplantation. Islet were isolated from donor pancreases according to clinical routine and infused simultaneously with thawed Tregs in the portal vein. Transplant outcomes with focus on safety was evaluated for three months.
Results
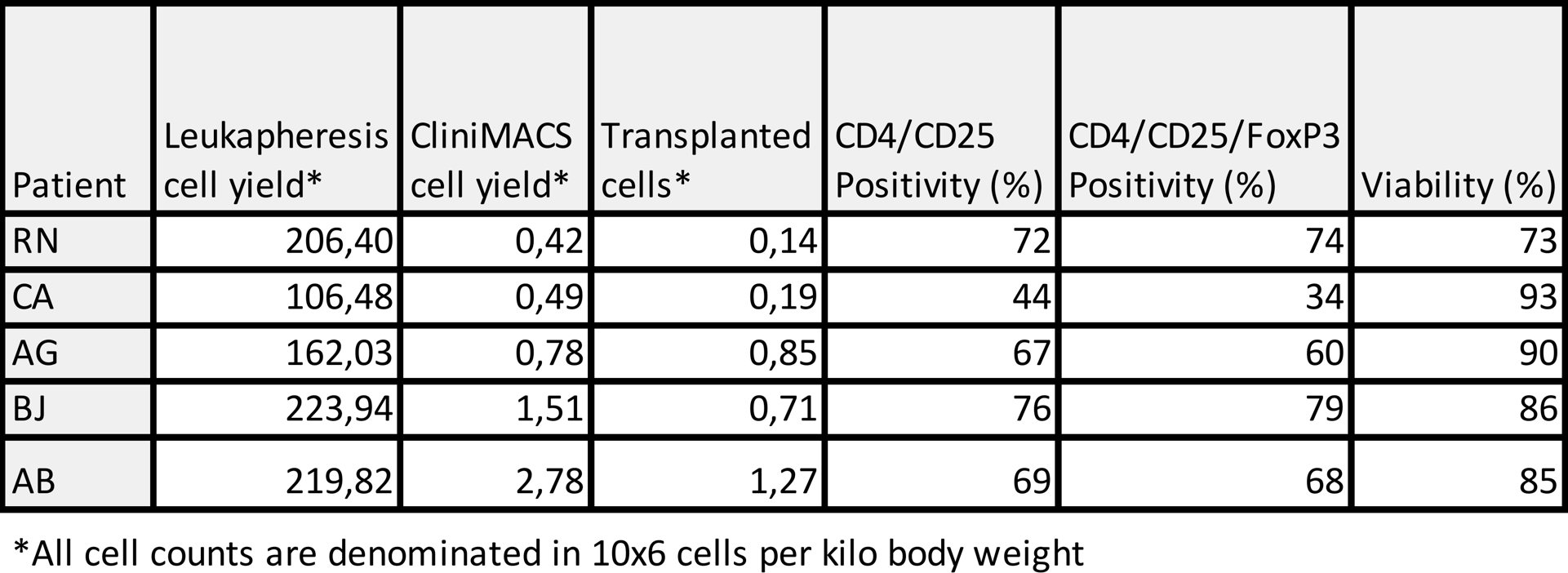
The only adverse events reported were related to the immunosuppressive drugs. Dose ranges of 0.14 to 1.27 x 106 T cells per kilo body weight were transplanted. No negative effects of these were seen neither in patients nor in islet function, regardless of dose. Clinical parameters were comparable to standard islet transplantation without Tregs.
Conclusion
This first-in-man study of autologous Treg infusion in allogenic pancreatic islet transplantation shows that the treatment is safe and feasible. Based on these results, future efficacy studies may be performed under the label of advanced therapeutic medical products (ATMP) using modified or expanded Tregs, with aim to reduce the need for general immunosuppression.